A) Li.
B) Na.
C) K.
D) Rb.
E) Cs.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct symbol for the element. -mercury
A) Au
B) Hg
C) Ag
D) Mc
E) Pb
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the noble gas in the following list.
A) gold
B) chlorine
C) helium
D) oxygen
E) nitrogen
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic mass of an element is equal to
A) its mass number.
B) the average mass of all of the naturally occurring isotopes of the element.
C) its atomic number.
D) one-twelfth of the mass of a carbon-12 atom.
E) a weighted average mass of all of the naturally occurring isotopes of the element.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The elements lithium, sodium, and potassium
A) have the same mass number.
B) have the same number of neutrons.
C) are isotopes of each other.
D) are in the same group.
E) are in the same period of elements.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct electron-dot structure for carbon?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element in this list with chemical properties similar to magnesium is
A) sodium.
B) chlorine.
C) boron.
D) strontium.
E) carbon.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass number of an atom can be calculated from
A) the number of neutrons.
B) the number of electrons.
C) the number of electrons plus protons.
D) the number of protons plus neutrons.
E) the number of protons.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider an isotope of sodium with a mass number of 25. The number of neutrons in this isotope of sodium is
A) 32.
B) 11.
C) 16.
D) 25.
E) 14.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of dots in the electron dot structure of nitrogen is
A) one.
B) two.
C) three.
D) four.
E) five.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the metalloid in the following list.
A) sulfur
B) copper
C) silver
D) fluorine
E) germanium
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the element with the electron configuration 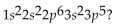
A) Cl
B) S
C) F
D) Ar
E) Be
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Chlorine has two naturally occurring isotopes. The isotope Cl-35 (mass = 35.0 amu) makes up 75.8% of the sample, and the isotope Cl-37 (mass = 37.0 amu) makes up 24.2% of the sample. What is the average atomic Mass for chlorine?
A) 36.6 amu
B) 35 amu
C) 36.0 amu
D) 35.521 amu
E) 35.5 amu
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many valence electrons are in the electron-dot structures for the elements in group 3A(13) ?
A) 1
B) 2
C) 3
D) 4
E) 6
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct symbol for the element. -potassium
A) Po
B) Pt
C) P
D) K
E) Ko
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The smallest particle of an element that retains the characteristics of the element is a(n)
A) neutron.
B) atom.
C) nucleus.
D) electron.
E) proton.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The maximum number of electrons that may occupy the third energy level is
A) 18.
B) 32.
C) 8.
D) 10.
E) 2.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Write in the electronic configuration for the atom shown. -Sulfur
Correct Answer

verified
Correct Answer
verified
Essay
Write in the electronic configuration for the atom shown. -Sodium
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Semiconductors are located in the periodic table on (or in) the
A) line dividing metals from nonmetals in the table.
B) left side of the table.
C) first period of the table.
D) last period of the table.
E) right side of the table.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 83
Related Exams