A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following compounds are constitutional isomers. Which can form hydrogen bonds with water?
A) ![]()
B) ![]()
C) ![]()
D) All can form hydrogen bonds with water.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the structures given below. 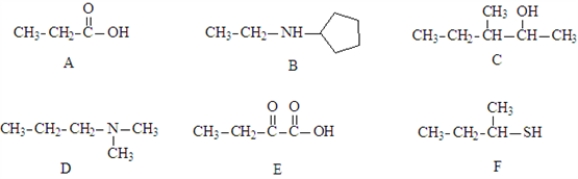 Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure__________________ and structure _______________ could react to form ethyldimethylammonium propanoate.
Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure__________________ and structure _______________ could react to form ethyldimethylammonium propanoate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the following substance? CH3-NH-CH2−CH2-CH3
A) methylpropylamine
B) propylmethylamine
C) aminobutane
D) butanamine
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Only amino acids can form zwitterions.
B) False
Correct Answer

verified
Correct Answer
verified
Essay
Draw the structure of sodium benzoate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When phenol dissolves in water, it functions as
A) a weak base
B) a weak acid
C) a neutral compound
E) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The amine shown below is a tertiary amine. 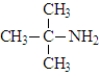
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The salt of propanoic acid and the sodium ion would be named as sodium propanoate.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the structures given below.  Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure____________________ would not be neutralized by the buffers found in the body.
Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure____________________ would not be neutralized by the buffers found in the body.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solutions is most likely to have the highest pH?
A) 0.010 M propanol
B) 0.010 M propanoic acid
C) 0.010 M propyl amine
D) 0.010 M phenol
E) 0.010 M propanethiol
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
In decarboxylation reactions, the carboxylic acid group produces carbon dioxide.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The ketone carbonyl group in the following compound is on the α carbon atom. 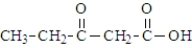
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The active ingredient in Prozacis shown below. 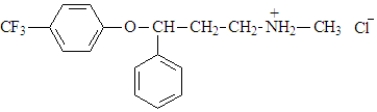 This compound:
This compound:
A) is a hydrochloride salt.
B) contains the conjugate acid of an amine.
C) was formed by the reaction of an amine and HCl.
D) all of the above
F) None of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the structures given below.  Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure ____________________ could be a hydrogen bond donor or acceptor in water.
Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure ____________________ could be a hydrogen bond donor or acceptor in water.
Correct Answer

verified
Correct Answer
verified
True/False
The structure of isoleucine is 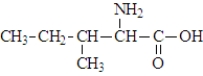 The zwitterion form of the amino acid isoleucine is
The zwitterion form of the amino acid isoleucine is 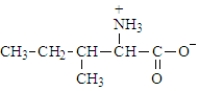
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following classes of amines can act as both a hydrogen bond acceptor and a hydrogen bond donor when dissolved in water?
A) primary
B) secondary
C) tertiary
D) both primary and secondary
E) primary, secondary and tertiary
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the formation of a thioester bond, the involved hydrogen atoms:
A) are transferred to FAD.
B) are transferred to NAD+.
C) remain with the thiol.
D) remain with the aldehyde.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solutions is most likely to have the lowest pH?
A) 0.010 M propanol
B) 0.010 M propanoic acid
C) 0.010 M propyl amine
D) 0.010 M phenol
E) 0.010 M propanethiol
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The following represents the general structure of a thioester. 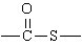
B) False
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 64
Related Exams